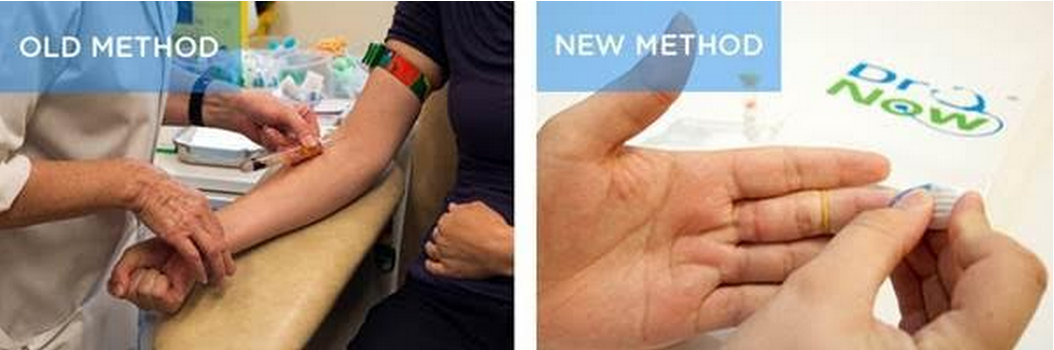
Dr Now, a UK-based medical diagnostic and delivery mobile platform from Now Healthcare Group, has unveiled a self-test ‘tumor marker’ kits to help diagnose ovarian cancer and assess the severity of potential future heart failure. The self diagnosis test kits allow patients to administer a thorough blood test themselves, simply by pricking their finger and providing a small blood sample. Results are then returned to the patient within 24 hours of the sample being received by the Dr Now lab.
How It Works
Tumour markers are substances that are produced by cells in response to cancer or certain benign conditions. They are produced at higher levels in cancerous conditions than normal, which makes them detectable through a simple blood test. The NT-Pro (BNP) test helps to diagnose and assess the severity of a potential heart failure, and can be effective up to 15 years before the heart fails. This preventative test can allow those at risk to receive the necessary treatment to minimize the risk of a future heart problem. The CA125 blood test is used to diagnose ovarian cancer. It detects above-normal levels of the tumour marker CA125, while the related HE4 test considers the risk factor of malignancy.
These new tests offered by Dr Now complements the company’s current diagnosis and medication delivery service offered to patients directly from an app-based video consultation with an MRCGP-certified GP. Currently in a ‘soft launch’ phase, the Dr Now platform will be fully launched in October 2015. The Dr Now app is now available to download for iOS and Android devices from the App Store and Google Play.
