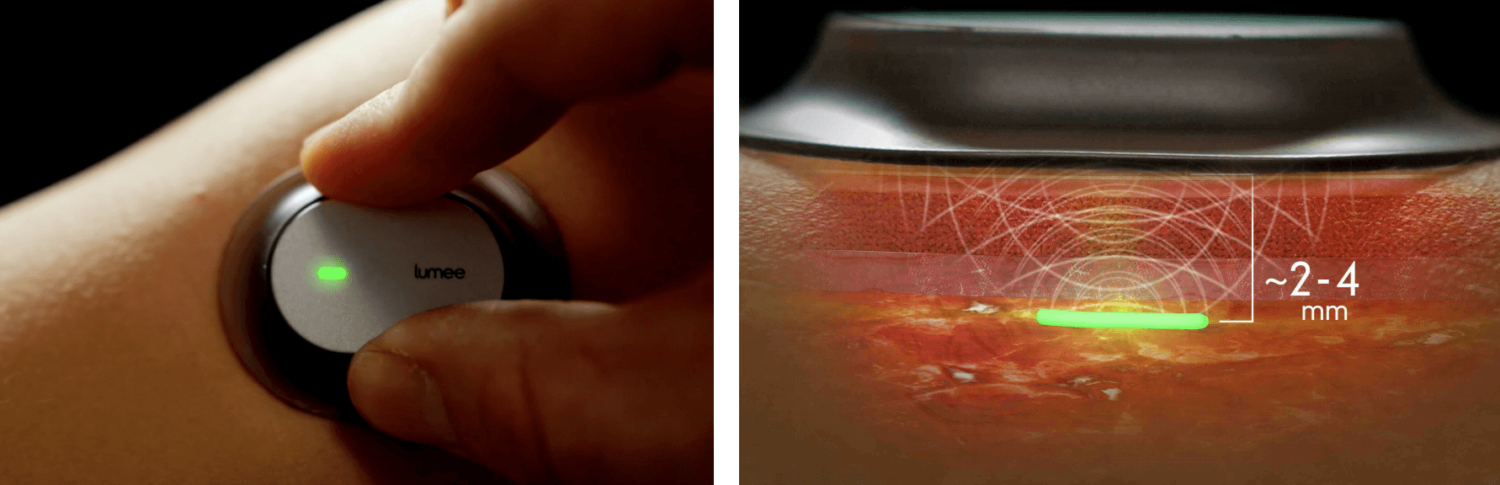
Today at FLEX 2019, integrated biosensor makers Profusa and NextFlex unveiled a skin-worn reader prototype with an injected hydrogel biosensor for continuous oxygen monitoring in tissue. The Lumee™ Oxygen Platform is intended for use in patients with potential acute and/or chronic changes in tissue oxygen levels who may benefit from continuous monitoring.
Lumee Oxygen Hydrogel Biosensor
The Lumee Oxygen Hydrogel biosensor, a tiny flexible fiber (3 mm to 5 mm long and approximately 500 microns in diameter) that is placed under the skin with a specially designed injector. The Lumee optical reader measures oxygen levels in the surrounding tissue by exciting the biosensor with light and then measuring the emitted fluorescent light. Profusa’s biosensors fully integrate with the body’s tissue without any metal devices or electronics, avoiding the foreign body response for up to two years. The injectable hydrogel sensor is currently CE Marked in Europe and it is not yet commercially available elsewhere.
Skin-Worn Reader Prototype Details
The skin-worn reader was developed in record time, just in four months, at NextFlex’s research center and fab in San Jose, Calif., since the facility and the team combine expertise in flexible hybrid electronics, engineering, materials science and optics all reside under one roof. NextFlex has taken Profusa’s design work and criteria and pushed them toward a smaller, flexible reality that works in harmony with Profusa’s tissue sensors. With this stage completed and other medical devices in the pipeline, NextFlex is engaged in the application for FDA approval for medical device manufacturing.
“Ten years ago, you couldn’t build a reader that could sense multiple signals and filter out the ‘noise’ of the body’s other signals, all while being small and skin-worn,” said Ben Hwang, CEO of Profusa. “With the latest advancements in flexible hybrid electronics technology, it’s just mature enough to work the way we want — primarily because we selected the right domain expertise partner to work with, namely NextFlex. Rather than managing development across multiple R&D and manufacturing facilities, we found a single place at NextFlex’s Technology Hub, where we could develop our solution in very short time and have the right conversations to move our reader design toward production at scale.”
